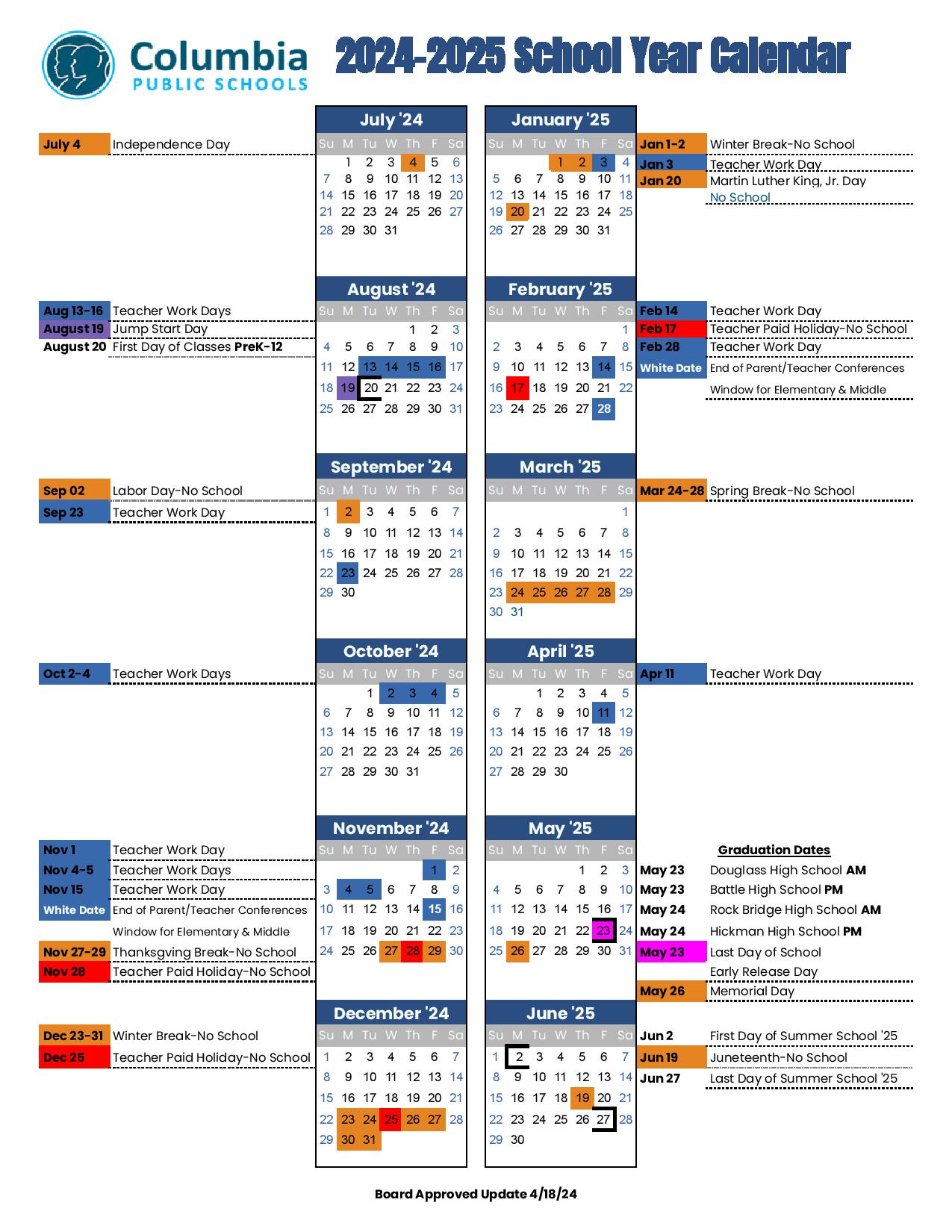
12+ Key Elements To Master Gauge Pressure Formula Easily

Understanding gauge pressure is fundamental in various fields, including physics, engineering, and chemistry. Gauge pressure refers to the pressure of a system relative to the surrounding atmospheric pressure. It’s a critical concept because it helps in calculating the total pressure of a system, which is essential for designing and operating equipment safely and efficiently. The formula to calculate gauge pressure is relatively straightforward, but mastering it involves understanding several key elements. Here’s a comprehensive breakdown to help you grasp the gauge pressure formula with ease.
Definition and Formula
Gauge pressure (P_gauge) is defined as the pressure above or below the atmospheric pressure (P_atm). The formula to calculate gauge pressure is:
P_gauge = P_absolute - P_atm
Where: - P_gauge is the gauge pressure, - P_absolute is the absolute pressure, and - P_atm is the atmospheric pressure.
Atmospheric pressure at sea level is approximately 101.3 kPa (kiloPascals) or 14.7 psi (pounds per square inch).
Understanding Absolute Pressure
Absolute pressure (P_absolute) is the total pressure in a system, including the atmospheric pressure. It’s measured relative to a perfect vacuum, where the pressure is zero. Understanding absolute pressure is crucial because it allows you to calculate the gauge pressure accurately.
Importance of Atmospheric Pressure
Atmospheric pressure (P_atm) is the pressure exerted by the weight of air in the atmosphere. It varies with altitude, weather conditions, and temperature. For most calculations at sea level, using the standard value of 101.3 kPa or 14.7 psi is sufficient. However, in applications at different altitudes or where precision is critical, the actual atmospheric pressure must be measured or calculated.
Practical Applications
Gauge pressure has numerous practical applications: - Tire Pressure: Car tires are inflated to a certain gauge pressure to ensure safety and efficiency. Underinflated tires can lead to accidents and decreased fuel efficiency. - Medical Equipment: In medical settings, understanding gauge pressure is crucial for equipment like ventilators and infusion pumps. - Industrial Processes: Gauge pressure is vital in chemical processing, power generation, and oil refining, where precise pressure control is necessary.
Calculation Examples
To illustrate how the formula works, let’s consider an example: - Suppose the absolute pressure in a tank is 150 kPa, and the atmospheric pressure is 101.3 kPa. - The gauge pressure (P_gauge) would be 150 kPa - 101.3 kPa = 48.7 kPa.
Another example, using psi: - If the absolute pressure is 30 psi and the atmospheric pressure is 14.7 psi, then the gauge pressure is 30 psi - 14.7 psi = 15.3 psi.
Tips for Mastery
- Understand the Context: Always consider the context in which pressure is being measured. Whether it’s absolute or gauge pressure, this distinction is crucial for accurate calculations.
- Convert Units Correctly: Be comfortable converting between different units of pressure (e.g., kPa to psi) to ensure consistency in your calculations.
- Practice with Different Scenarios: The more you practice calculating gauge pressure in various scenarios, the more intuitive it becomes.
- Remember the Formula: While simple, the formula P_gauge = P_absolute - P_atm is foundational. Ensure you understand and can apply it correctly.
- Consider Atmospheric Pressure Variations: Especially in applications where small changes in pressure can have significant effects, accounting for variations in atmospheric pressure is vital.
Common Mistakes to Avoid
- Incorrectly Assuming Atmospheric Pressure: Always verify the atmospheric pressure value you are using, especially if your application is not at sea level or under standard conditions.
- Mixing Up Absolute and Gauge Pressure: Be clear about which type of pressure you are dealing with to avoid errors in your calculations.
- Not Converting Units Properly: Ensure that all values are in the same units before performing calculations to avoid conversion errors.
Conclusion
Mastering the gauge pressure formula is about understanding its components and applications. By recognizing the importance of absolute pressure, atmospheric pressure, and the distinctions between them, you can easily calculate gauge pressure and apply it in various contexts. Remember, practice and a thorough understanding of the principles behind the formula are key to becoming proficient in calculating and applying gauge pressure in real-world scenarios.
What is the primary difference between absolute and gauge pressure?
+Absolute pressure is the total pressure in a system, including atmospheric pressure, measured relative to a perfect vacuum. Gauge pressure, on the other hand, is the pressure above or below the atmospheric pressure.
How do you calculate gauge pressure?
+Gauge pressure is calculated by subtracting the atmospheric pressure from the absolute pressure: P_gauge = P_absolute - P_atm.
What is the standard atmospheric pressure at sea level?
+The standard atmospheric pressure at sea level is approximately 101.3 kPa or 14.7 psi.
By following these guidelines and practicing with real-world examples, you’ll find that mastering the gauge pressure formula becomes second nature, enabling you to tackle complex problems with confidence.