How To Find Atomic Radius
Understanding the atomic radius is crucial in chemistry as it helps in understanding the size of an atom, which in turn, influences the chemical properties of an element. The atomic radius is defined as the distance from the nucleus of an atom to the edge of the electron cloud. However, since the edge of the electron cloud is not a well-defined boundary, the atomic radius is typically measured as the distance from the nucleus to the point where the electron density falls to a certain threshold. Here’s how to find the atomic radius of an element:
Method 1: Empirical Method
One of the most common methods to determine the atomic radius is through empirical methods, which involve experimentally measuring the distances between atoms in a crystal lattice. This method is based on the assumption that the distance between the centers of two adjacent atoms in a crystal is equal to the sum of their atomic radii.
- X-Ray Diffraction: One of the empirical methods to find the atomic radius involves using X-ray diffraction. In this technique, X-rays are scattered by the electrons in the crystal lattice, producing a diffraction pattern that can be used to determine the interatomic distances.
- Electron Diffraction: Similar to X-ray diffraction, electron diffraction can also be used to determine the atomic radius by analyzing the diffraction pattern produced when a beam of electrons is passed through a crystal.
Method 2: Theoretical Calculations
Theoretical calculations can also be used to estimate the atomic radius. These methods involve solving the Schrödinger equation for the atom to obtain the electron density distribution, from which the atomic radius can be calculated.
- Hartree-Fock Method: This is a computational method used in quantum chemistry to determine the electronic structure of atoms and molecules. By solving the Hartree-Fock equations, one can obtain the electron density distribution and estimate the atomic radius.
- Density Functional Theory (DFT): DFT is another computational method that can be used to estimate the atomic radius. It is based on the principle that the ground-state density of an atom or molecule determines its electronic structure.
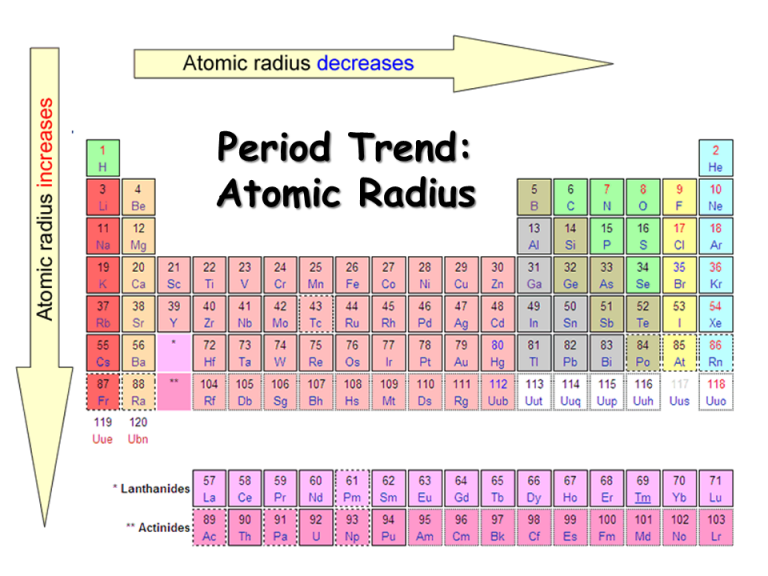
Method 3: Trends in the Periodic Table
While not a direct method for calculating the atomic radius, understanding the trends in the periodic table can provide valuable insights into how the atomic radius changes across different elements.
- Decrease Across a Period: As one moves from left to right across a period in the periodic table, the atomic number increases, which means the number of protons in the nucleus increases. This increase in positive charge attracts the electrons more strongly, causing the atomic radius to decrease.
- Increase Down a Group: Moving down a group in the periodic table, the number of electron shells increases, which results in an increase in the atomic radius. This is because each additional shell adds to the distance from the nucleus to the outermost electron.
Conclusion
Finding the atomic radius involves a combination of experimental measurements and theoretical calculations. Understanding the trends in the periodic table can also provide insights into how the atomic radius varies across different elements. Each method has its own set of assumptions and limitations, and the choice of method depends on the specific context and the desired level of accuracy.
FAQ Section
What is the importance of knowing the atomic radius of an element?
+Knowing the atomic radius of an element is important because it influences the chemical properties of the element, such as reactivity and the ability to form compounds. It also helps in understanding the structure of molecules and crystals.
How does the atomic radius change across the periodic table?
+The atomic radius generally decreases across a period from left to right due to the increasing positive charge in the nucleus, and it increases down a group due to the addition of new electron shells.
What are the limitations of theoretical methods for calculating the atomic radius?
+Theoretical methods such as the Hartree-Fock method and Density Functional Theory (DFT) are limited by their underlying assumptions and the complexity of the systems they can handle. They may not always accurately predict the atomic radius, especially for larger atoms or complex molecules.
Final Thoughts
Understanding and calculating the atomic radius is a complex task that involves both empirical measurements and theoretical calculations. By combining these approaches and understanding the trends in the periodic table, scientists can gain a deeper understanding of the properties of elements and their potential applications in various fields.

