Ocl2 Lewis Structure Explained: Bonds Revealed

The ocl2 lewis structure is a fundamental concept in chemistry that helps us understand the bonding and molecular geometry of this compound. To start, let’s break down the elements involved. Ocl2, or oxychloride, consists of one oxygen atom and two chlorine atoms. Understanding the lewis structure is crucial because it reveals how the atoms share electrons to form bonds, which in turn determines the shape and properties of the molecule.
Understanding the Basics of Lewis Structures
Lewis structures are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist. They are a crucial tool for understanding the properties and behavior of molecules. The basic steps to drawing a lewis structure include determining the total number of valence electrons, drawing a skeleton structure, and then filling in the electrons to satisfy the octet rule for each atom, which states that an atom will try to have eight electrons in its outer shell.
Constructing the Ocl2 Lewis Structure
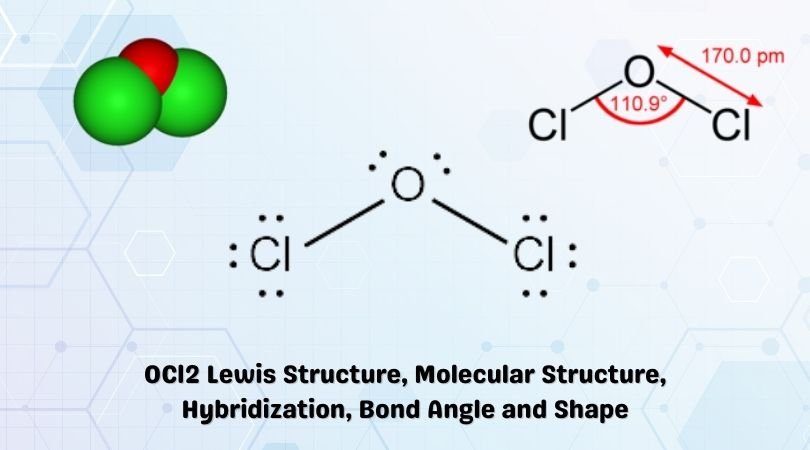
To construct the ocl2 lewis structure, we start by determining the total number of valence electrons. Oxygen has six valence electrons, and each chlorine atom has seven, giving us a total of 20 valence electrons (6 + 7 + 7).
Next, we draw a skeleton structure where the oxygen atom is bonded to the two chlorine atoms. Given oxygen’s higher electronegativity, it is typically the central atom in such structures. We then use single bonds to connect the oxygen to each chlorine, which uses 4 of our electrons (2 for each bond), leaving us with 16 electrons to distribute.
After forming the single bonds, we distribute the remaining electrons around the atoms to satisfy the octet rule. Each chlorine atom already has two electrons from the single bond with oxygen, so we add six more electrons to each chlorine (three pairs) to complete their octets. This leaves us with 4 electrons, which we place on the oxygen atom as two lone pairs, thus completing its octet as well.
Bonds Revealed: Analyzing the Structure
The bonds in ocl2 are primarily covalent, with the oxygen and chlorine atoms sharing electrons to form the single bonds. The lewis structure we’ve drawn indicates two single bonds between oxygen and chlorine and two lone pairs on the oxygen atom. However, to fully understand the bonding, we must consider the molecular orbital theory, which suggests the involvement of both sigma and pi bonds in the molecule. The combination of these bonds results in a bent or V-shape molecular geometry due to the presence of the lone pairs on the oxygen atom, which exert repulsive forces on the bonding pairs, pushing the chlorine atoms closer together.
Molecular Geometry and Polarity
The molecular geometry of ocl2 is bent due to the lone pairs on the oxygen atom, which causes the molecule to have a net dipole moment. This bent shape is a result of the VSEPR (Valence Shell Electron Pair Repulsion) theory, which states that electron pairs around a central atom will arrange themselves to minimize repulsions. The polarity of ocl2 arises from the difference in electronegativity between oxygen and chlorine, with oxygen being more electronegative. This polarity is crucial for understanding the chemical properties and reactivity of ocl2.
Practical Applications and Chemical Properties
Ocl2, or more specifically compounds with similar structures, have various applications in chemistry and industry. For example, oxychlorides are used in the production of various chemicals and can act as intermediates in synthesis reactions. Understanding their lewis structures and bonding helps predict their chemical behavior and reactivity, which is essential for designing and optimizing chemical processes.
Conclusion
In conclusion, the ocl2 lewis structure provides valuable insights into the molecular structure and bonding of oxychloride compounds. By understanding how the atoms share electrons and the geometry that results from these interactions, we can better predict the chemical properties and potential applications of such compounds. This knowledge is fundamental in chemistry and is used to design new compounds with specific properties and to understand the reactivity of existing ones.
What is the significance of understanding the lewis structure of ocl2?
+Understanding the lewis structure of ocl2 is significant because it helps in predicting the molecular geometry, polarity, and reactivity of the compound. This knowledge is crucial for designing new compounds and understanding the chemical properties of existing ones.
How does the presence of lone pairs affect the molecular geometry of ocl2?
+The presence of lone pairs on the oxygen atom in ocl2 causes the molecule to have a bent or V-shape geometry. The lone pairs exert repulsive forces on the bonding pairs, pushing the chlorine atoms closer together and resulting in a bent shape.
What role does electronegativity play in determining the polarity of ocl2?
+Electronegativity plays a crucial role in determining the polarity of ocl2. The difference in electronegativity between oxygen and chlorine, with oxygen being more electronegative, contributes to the net dipole moment of the molecule, making it polar.
Understanding the intricacies of chemical bonding and molecular structure, as seen in the ocl2 lewis structure, is vital for advancing our knowledge of chemistry and developing new applications and technologies. By exploring these fundamental concepts, we can unlock deeper insights into the behavior of molecules and their potential uses.

